About Us
Valent BioSciences is a global leader in the research, development, and commercialization of highly effective, low-risk, and environmentally compatible technologies and products for agriculture, public health, and forest health.

Delivering innovation to every corner of the globe
Through the power of fermentation and microbiology, Valent BioSciences develops biorational products that create value and solve problems for customers around the world. These products include environmentally compatible bioinsecticides and plant growth regulators that are naturally occurring or chemically derived, and are used in sustainable systems.
Our best-in-class technology assessment, formulation expertise, development experience, and product quality are trusted by our customers and industry peers alike.
Valent BioSciences is a wholly owned subsidiary of Sumitomo Chemical Co., Ltd., a global leader in developing creative solutions for health and crop sciences. For more than a century, Sumitomo Chemical has been at the cutting edge of solving problems that face the global community.
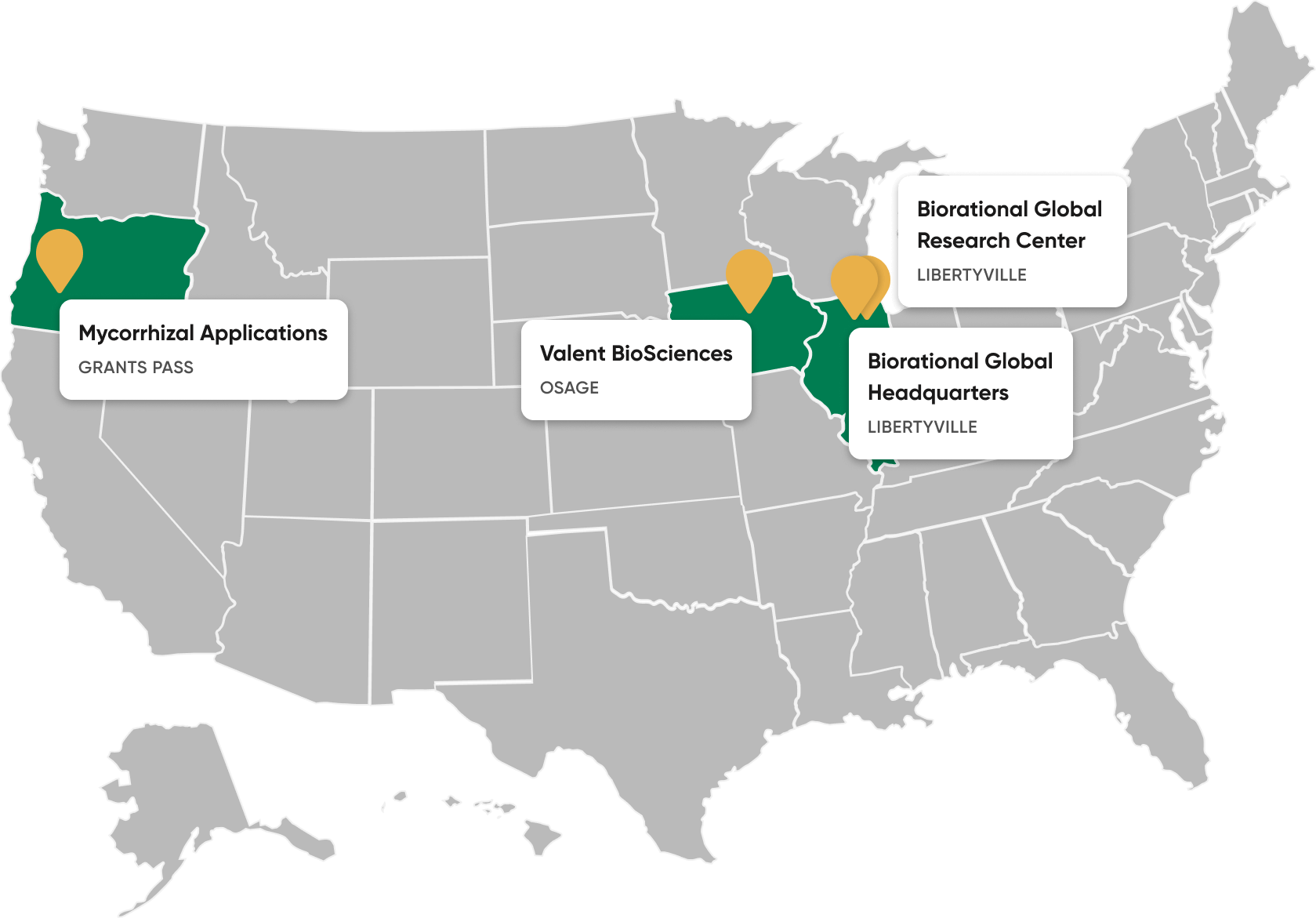

Our Facilities
Our more than 90,000-square-foot research facility in Libertyville, Illinois, and our 130,000 square-foot biorational manufacturing facility in Osage, Iowa — the largest purpose-built biorational facility in the world — enable us to innovate and create efficacious biorational products.
Our Sales Affiliates

To deliver our biorational solutions to customers in a timely and efficient manner, Valent BioSciences works with numerous regional sales affiliates around the world.
These sales affiliates are dedicated to distributing not only Valent BioSciences’ biorational products, but conventional products from Sumitomo Chemical and other companies as well.
Our solutions create an impact around the world
worldwide registrations
countries
sales affiliates
Corporate Social Value

As a global leader in the development and commercialization of biorational products, Valent BioSciences has a strong commitment to both sustainability and corporate social value. Being a responsible corporate citizen has been ingrained in our culture ever since the company’s founding. Today, it remains at the forefront of everything we do.
Our Company Values
At Valent BioSciences, we look to our company values to guide us in every decision we make, from research to production and beyond.
Connectedness fosters collaboration, inclusion, and strong relationships within teams and with external partners. It ensures that we work together effectively to achieve our goals by leveraging our diverse talents and resources..
Innovation is the pursuit of new ideas, solutions, and methods that improve our products, services, and processes. It drives progress and ensures that we stay ahead of market trends and customer needs.
Integrity means acting with honesty, transparency, and ethical standards in all business activities, ensuring that our actions and decisions are trustworthy.



Frequently Asked Questions
Biorationals are low-impact substances or products that are typically biologically derived. If biorationals are synthetic, they are structurally similar and functionally identical to biologically occurring material (with minor differences between the respective stereochemical isomer ratios derived from biological or synthetic origins).
Biorationals include biopesticides and non-pesticidal products that have multiple uses, including crop stress management, enhanced plant physiology benefits, root growth management, and postharvest treatments, or as an alternative control agent to conventional pesticides and antimicrobials.
Source: ASTM, April 2013
Pest control products have gone through a great deal of scrutiny in recent years. Governments have limited the use of conventional chemistries that have been part of crop protection programs while at the same time, today’s growers are expected to produce more food than ever.
Valent BioSciences’ products offer growers solutions that can increase productivity with minimal impact to workers or the environment. Our products include a low risk profile and help maintain beneficial insect populations such as pollinators. Biorationals also help sustain the efficacy of today’s conventional chemistries, which may be prone to the onset of resistance when overused.
In the public health and forest health industries, the same issues exist around traditional chemistries. But biorationals from Valent BioSciences have little to no impact on non-target organisms and humans while targeting specific pests.
The Valent group of companies includes Valent BioSciences, Valent U.S.A., and Mycorrhizal Applications. Each of these sister companies is under the parent company, Sumitomo Chemical Co. Ltd., a leading Tokyo-based chemical company.
Valent BioSciences is a global technology company focused on agriculture, public health, and forest health solutions.
Valent U.S.A. markets and sells a full portfolio of products in the U.S. agricultural market, including biorationals from Valent BioSciences.
Mycorrhizal Applications provides growers with efficient, effective mycorrhizal fungi-based solutions.
Visit the following pages to learn more: Research & Development and About Us.